
Isabella Santos Foundation Grants $100,000 to Levine Children’s for Pediatric Cancer Trial
Isabella Santos Foundation Grants $100,000 to Levine Children’s for Pediatric Cancer Trial
Written by Rachel Wood, Director of Marketing
For Isabella’s 16th birthday in March, we set a big fundraising goal and celebrated her legacy by lighting luminaries in honor of cancer warriors everywhere. The support was overwhelming, the night was breathtaking. Through our Star Light Star Bright Luminary event, you helped us raise an unbelievable $91,327.
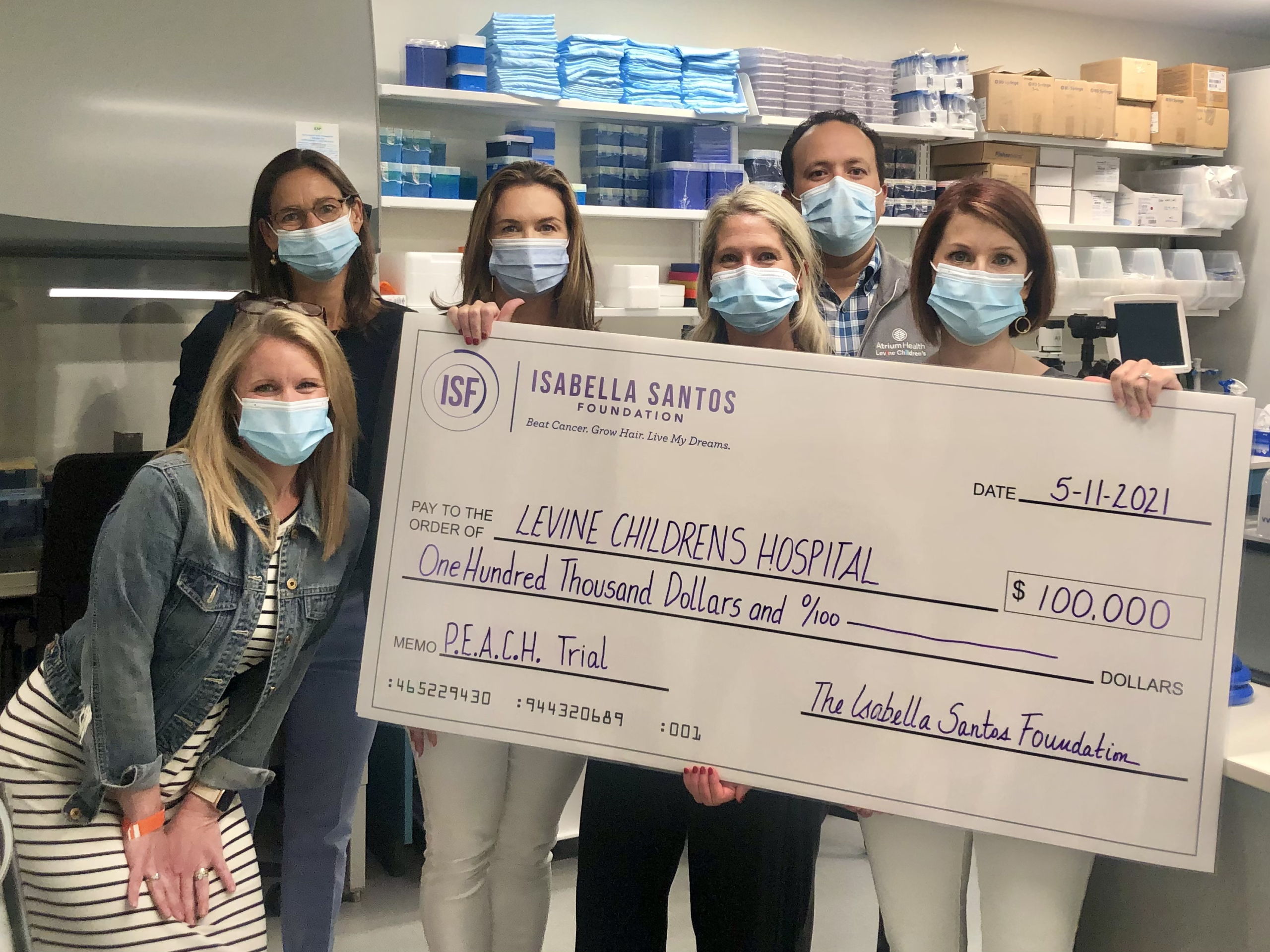 Today our team had the opportunity to take the Luminary event proceeds and present a check for a $100,000 grant to Dr. Sholler and Dr. Oesterheld at Levine Children’s Sholler pediatric cancer research lab. This grant will help fund Dr. Sholler’s P.E.A.C.H. Protocol, a clinical trial focused on relapsed neuroblastoma and newly diagnosed DIPG. Your donations are fast at work as patient enrollment starts in just a few months.
Today our team had the opportunity to take the Luminary event proceeds and present a check for a $100,000 grant to Dr. Sholler and Dr. Oesterheld at Levine Children’s Sholler pediatric cancer research lab. This grant will help fund Dr. Sholler’s P.E.A.C.H. Protocol, a clinical trial focused on relapsed neuroblastoma and newly diagnosed DIPG. Your donations are fast at work as patient enrollment starts in just a few months.
We have always dreamed of bringing childhood cancer research to Charlotte and now it’s happening right here at Levine Children’s. With the opening of the Sholler Pediatric Cancer Research Lab at Levine Children’s, the newest therapies and clinical trials for kids with rare and solid tumors are being developed in our backyard. Words cannot express how grateful we are that you are making this dream a reality.
Special thanks to our co-presenting sponsors Lake Norman Chrysler|Dodge|Jeep|Ram & Scott Clark Auto Group. We are overwhelmed by your support and how the Charlotte automotive community stepped in to make our Luminary event a shining success!
P.E.A.C.H. PROTOCOL: Precision mEdicine and Adoptive Cellular tHerapy for the treatment of recurrent neuroblastoma and newly diagnosed DIPG.
The P.E.A.C.H. Protocol is a Phase I, multicenter study, to evaluate treating children with recurrent neuroblastoma and newly diagnosed DIPG with molecular targeted therapy in combination with an anti-cancer immunotherapy vaccine. In addition, this trial will study the activity of the patients’ immune system and determine if it is affected by this therapy. Patient tumor cell lines and Xenograft models will be established & characterized. These will be used for future research and evaluated for drug response. The trial consists of two arms: neuroblastoma or DIPG. ISF funding will help support the advancement of the neuroblastoma arm of the clinical trial.
Despite efforts to develop novel treatment strategies, relapsing neuroblastoma and newly diagnosed DIPG continue to have poor prognoses and limited overall survival. Precision medicine and cell-based immunotherapies represent a promising treatment.
This clinical trial will evaluate treating children with recurrent neuroblastoma and newly diagnosed DIPG with molecular targeted therapy in combination with an anti-cancer immunotherapy vaccine. Targeted molecular therapy is a type of personalized medical therapy designed to treat cancer by interrupting unique molecular abnormalities that drive cancer growth. This type of therapy will be given in combination with an immunotherapy called dendritic cell (DC) and activated T-cell infusion. The outcome is to advance precision medicine for this group of patients while learning more about the effect of patients’ immune systems and its ability to target and eliminate a cancer. This clinical trial consists of four parts:
- Biopsy
- Standard Therapy (focal radiotherapy or chemotherapy)
- Molecular Guided Therapy (MGT)
- Immunotherapy (dendritic cell vaccine and activated T-cell infusion)




